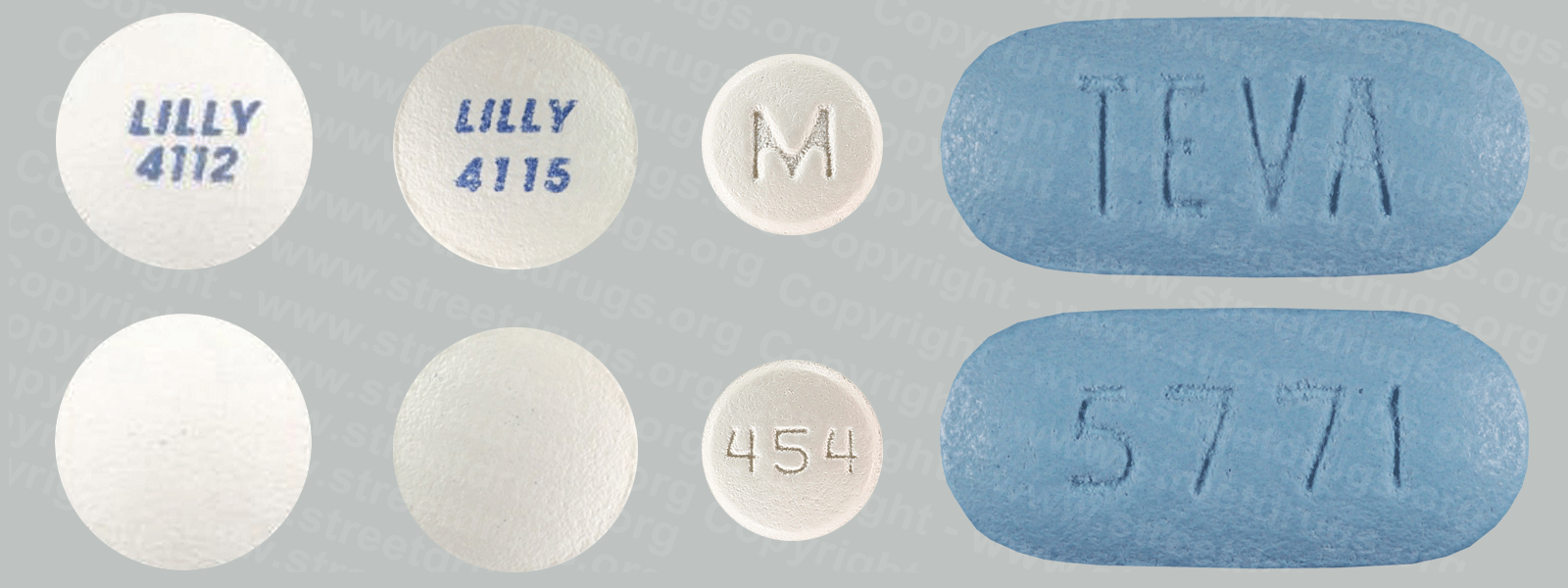
Chloral Hydrate
The oldest of the hypnotic (sleep inducing} depressants, chloral hydrate was first synthesized in 1832. Marketed as syrups or soft gelatin capsules, chloral hydrate takes effect in a relatively short time (30 minutes) and will induce sleep in about an hour. A solution of chloral hydrate and alcohol constituted the infamous “knockout drops” or “Mickey Finn.” Chloral Hydrate has a very limited use today.
At therapeutic doses, chloral hydrate has little effect on respiration and blood pressure; however; a toxic dose produces severe respiratory depression and very low blood pressure. Chronic use is associated with liver damage and a severe withdrawal syndrome. Although some physicians consider chloral hydrate to be the drug of choice for sedation of children before diagnostic, dental, or medical procedures, its general use as a hypnotic has declined. Chloral hydrate (Noctec® and other) and compounds, preparations, or mixtures containing chloral hydrate are in Schedule IV of the CSA. SIGNS OF OVERDOSE: Confusion (continuing); convulsions (seizures); difficulty in swallowing; drowsiness (severe); low body temperature; nausea, vomiting, or stomach pain (severe); shortness of breath or troubled breathing; slow or irregular heartbeat; slurred speech; staggering; weakness (severe).
Chloral hydrate is illegal without a prescription and is a schedule IV controlled substance in the United States. Its properties have sometimes led to its use as a date rape drug.[21][unreliable source?] This drug is still available in the United States, albeit it is relatively uncommon and not often kept in the inventory of major pharmacies. It has largely been abandoned for the treatment of insomnia in favor of newer drugs such as the Z-drugs family, which includes zolpidem, zaleplon, zopiclone, and eszopiclone. A small number of medical practitioners continue to prescribe it to treat insomnia when all other more modern medications have failed. In the United States, it is commonly supplied in syrup form in a 500 mg/5mL concentration. It is also supplied in suppository form, through use of this method of administration is extremely rare.
It is not controlled in Canada except that a prescription is required to purchase the pharmaceutical forms. Possession without a prescription is not illegal and industrial trade is not regulated.
The United Kingdom does not consider chloral hydrate to be a controlled substance.
Chloral hydrate is a prescription-only medicine (POM) in the Netherlands, but possession without a valid prescription will result only in a seizure of the drug, not prosecution. Production, sale, and distribution are however punishable by law. It is not listed under the Dutch Opium Law, but when the intent is human consumption, it is covered by the Geneesmiddelenwet (Medicine Act).
Other Sources:
Chloral Hydrate
a sedative is used in the short-term treatment of insomnia (to help you fall asleep … Chloral hydrate comes as a capsule and liquid to take by mouth and as a suppository to insert …
https://www.nlm.nih.gov/medlineplus/druginfo/meds/a682201.html – Drugs and Supplements
Substance use disorder
… They include alcohol, barbiturates, benzodiazepines (Valium, Ativan, Xanax), chloral hydrate, and paraldehyde. Using these substances can lead to …
https://www.nlm.nih.gov/medlineplus/ency/article/001522.htm – Medical Encyclopedia
Treatments for Sleep Changes (Alzheimer’s Association)
… temazepam “Sleeping pills” such as zolpidem, zaleplon, and chloral hydrate “Atypical” antipsychotics such as risperidone, olanzapine, and quetiapine …
www.alz.org/alzheimers_disease_10429.asp – External Health Links
Drug abuse
… yellow jackets” Benzodiazepines (e.g. Valium, Ativan, Xanax) Chloral hydrateParaldehyde Signs and symptoms of excessive alcohol or …
https://www.nlm.nih.gov/medlineplus/ency/article/001945.htm – Medical Encyclopedia
Known and Probable Human Carcinogens (American Cancer Society)
Chloramphenicol alpha-Chlorinated toluenes (benzal chloride, benzotrichloride, benzyl …