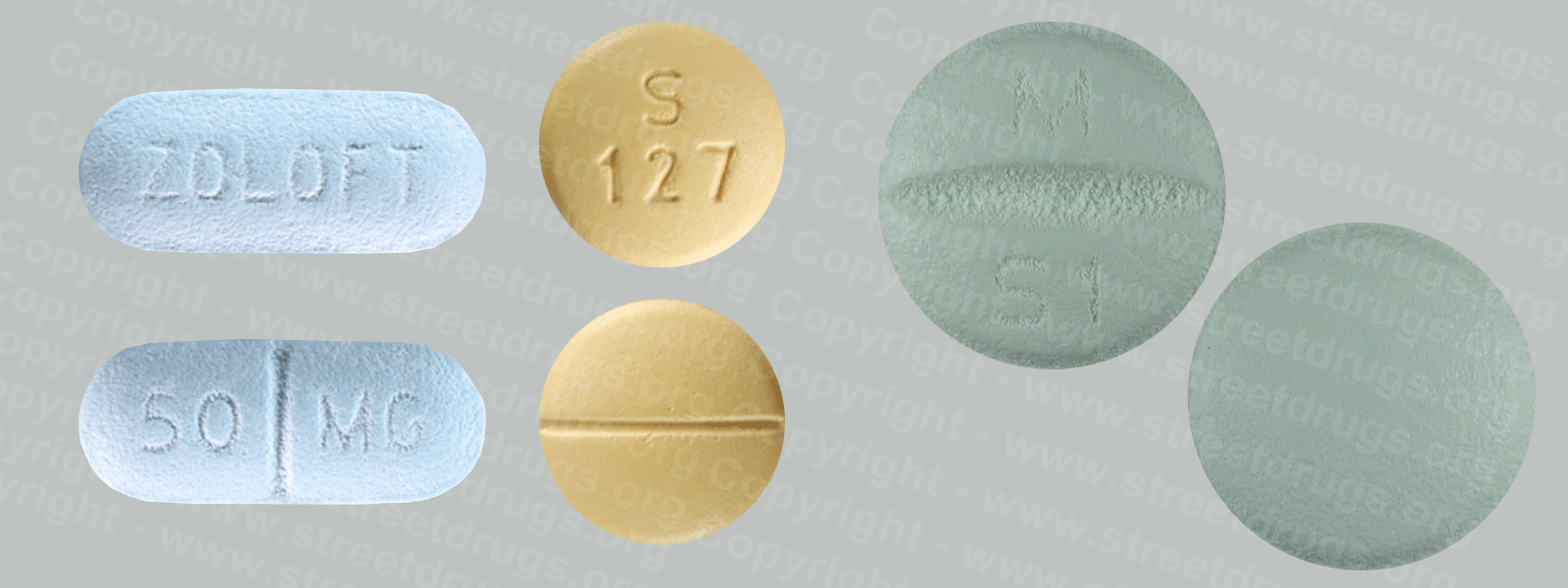
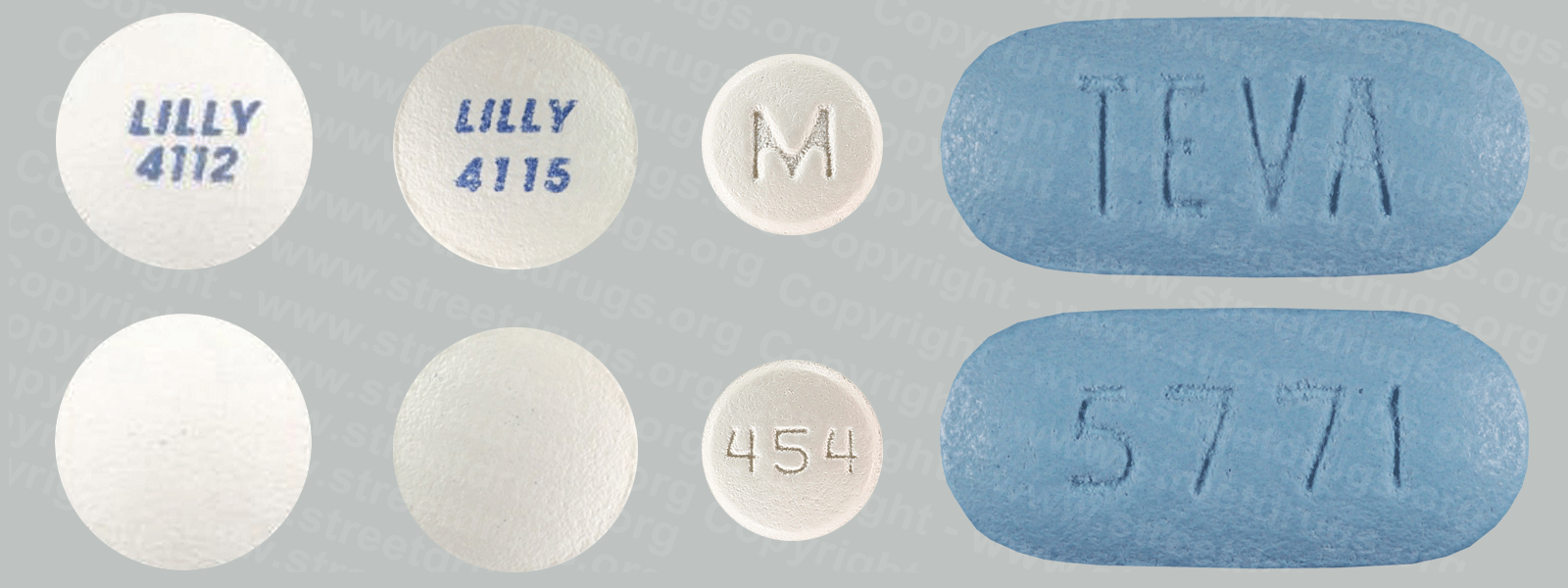
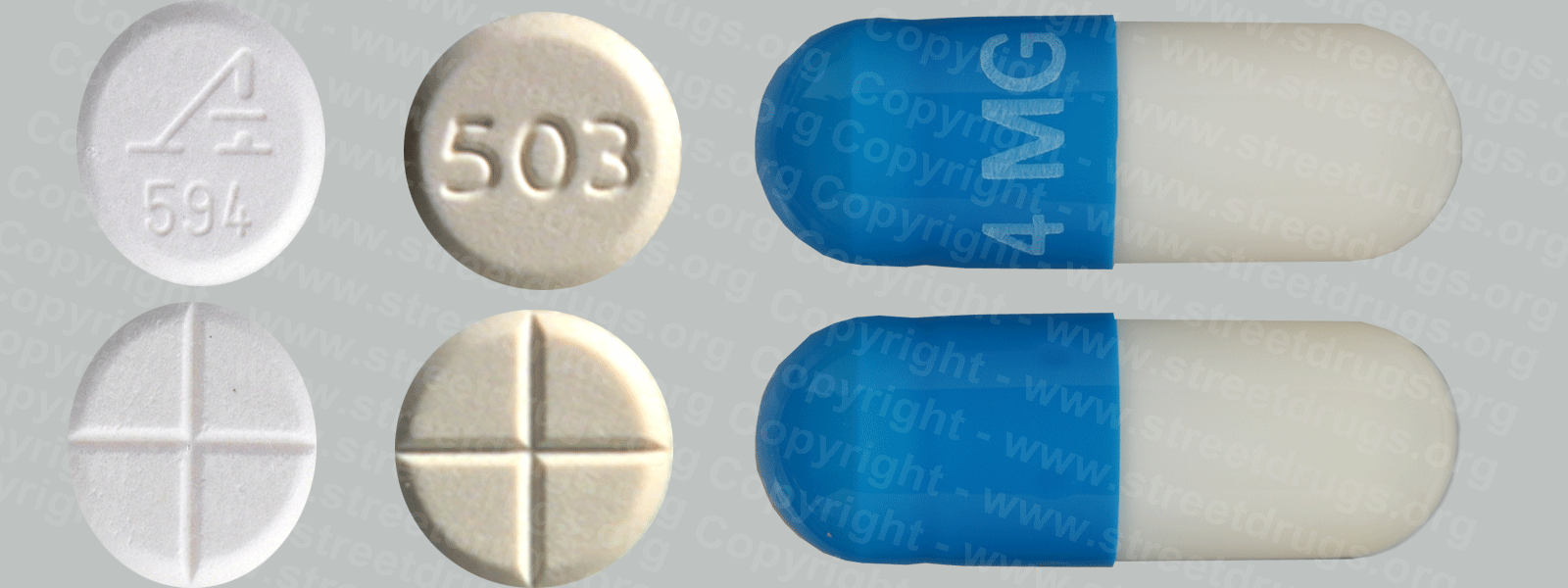
Fentanyl
Fentanyl was first synthesized in Belgium in the late 1950s, with an analgesic potency of about 80 to 100 times that of morphine, and introduced into medical practice in the 1960s as an intravenous anesthetic under the trade name of Sublimaze®. Thereafter; two other analogs were introduced; alfentanil (Alfenta®), an ultra-short (5-10 minutes) acting analgesic, and sufentanil (Sufenta®), an exceptionally potent analgesic (5 to 10 times more potent ) for use in heart surgery.
Today, fentanyl are extensively used for anesthesia and analgesia. Duragesic®, for example, is a transdermal patch used in chronic pain management, and Actiq® is a solid formulation of citrate on a stick that dissolves slowly in the mouth for transmucosal absorption. Actiq® is intended for opiate-tolerant individuals and is effective in treating breakthrough pain in cancer patients. Carfentanil (Wildnil®) is an analog of fentanyl with an analgesic potency 10,000 times that of morphine and is used in veterinary practice to immobilize certain large animals.
Illicit use of pharmaceutical fentanyl first appeared in the mid-1970s in the medical community and continues to be a problem in the United States. To date, over 12 different analogs of fentanyl have been produced clandestinely and identified in the U.S. drug traffic.
The biological effects of the fentanyl are indistinguishable from those of heroin, with the exception that the fentanyl may be hundreds of times more potent. Fentanyl is most commonly used by the intravenous administration, but like heroin, they may also be smoked or snorted.
Source: DEA
This narcotic drug has been diverted by pharmacy theft, fraudulent prescriptions, and illicit distribution by patients, physicians, and pharmacists. Theft has also been identified at nursing homes and other long-term care facilities. Oral transmucosal lozenges (Actiq®) are typically sold at $20-25 per unit or $450 per carton (contains 24 units) while transdermal patches (Duragesic®) are sold at prices ranging from $10 to $100 per patch depending upon the dose of the unit and geographical area. There is evidence of large illegal distribution rings selling fentanyl products along with other opioid pharmaceuticals.
Source: DEA Diversion Control Program Drop dead and suicide packets are street terms for fentanyl products.
The drug naloxone can reverse a fentanyl overdose when applied in time. However, because fentanyl is 50 times more potent than heroin, it may require several doses of naloxone to bring someone out of a fatal fentanyl overdose. Unfortunately, the person applying the naloxone will probably not know if fentanyl was present or how much fentanyl was consumed, and will probably not know that it is necessary to administer more naloxone to save the addicts life.
More info in the Prescription Drug Brochure
Other Sources:
- Transdermal Patch
- Fentanyl patches are used to relieve severe pain in people who are expected to need pain medication … and who cannot be treated with other medications.
- As a Narcotic
- This narcotic is used to treat breakthrough pain (sudden episodes of pain that occur despite round the clock … effects of the medication) to narcotic pain medications.
- Nasal Spray
- A nasal spray is used to treat breakthrough pain (sudden episodes of pain that occur despite round … effects of the medication) to narcotic pain medications.
- https://www.nlm.nih.gov/medlin
- Other SourcesDrug Identification GuideCDC FactsCDC Addiction InfoCDC Overdose StatsCDC Substance TreatmentWhitehouse Drug Free CommunitiesSamhsa Workplace ProgramsDrug FreeFederal Register Codification
CONTACT US
MANUFACTURER LIST