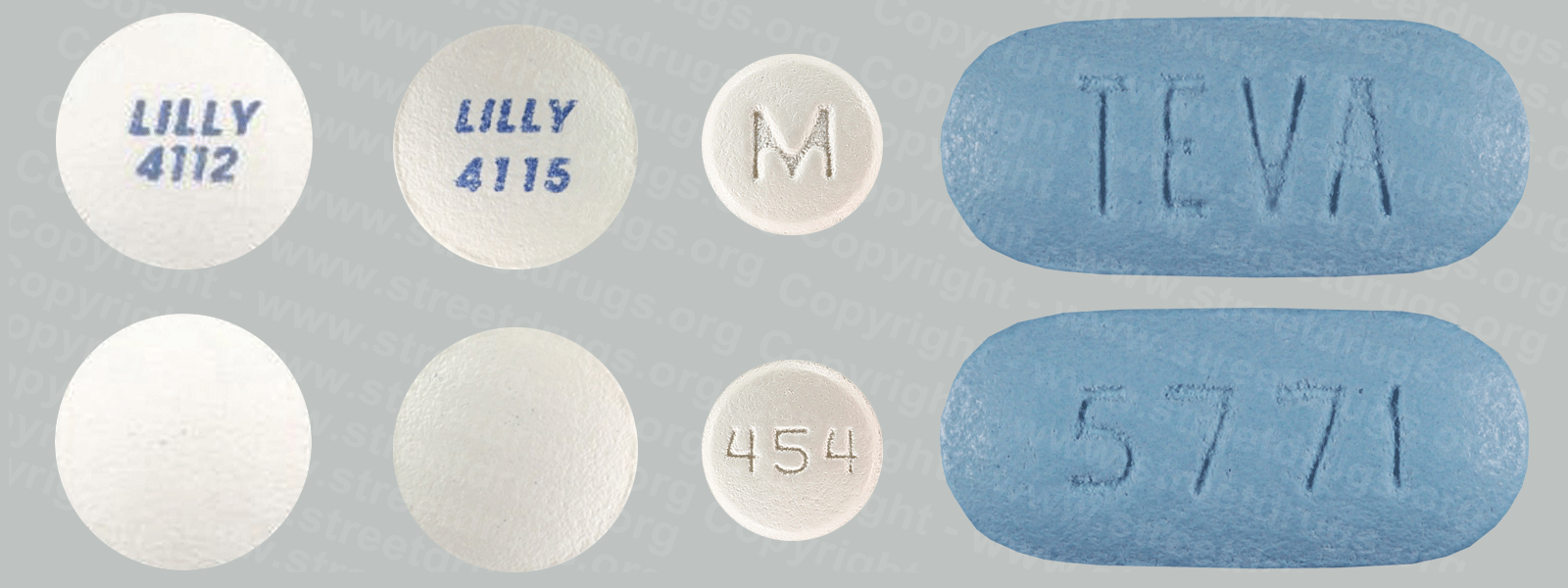
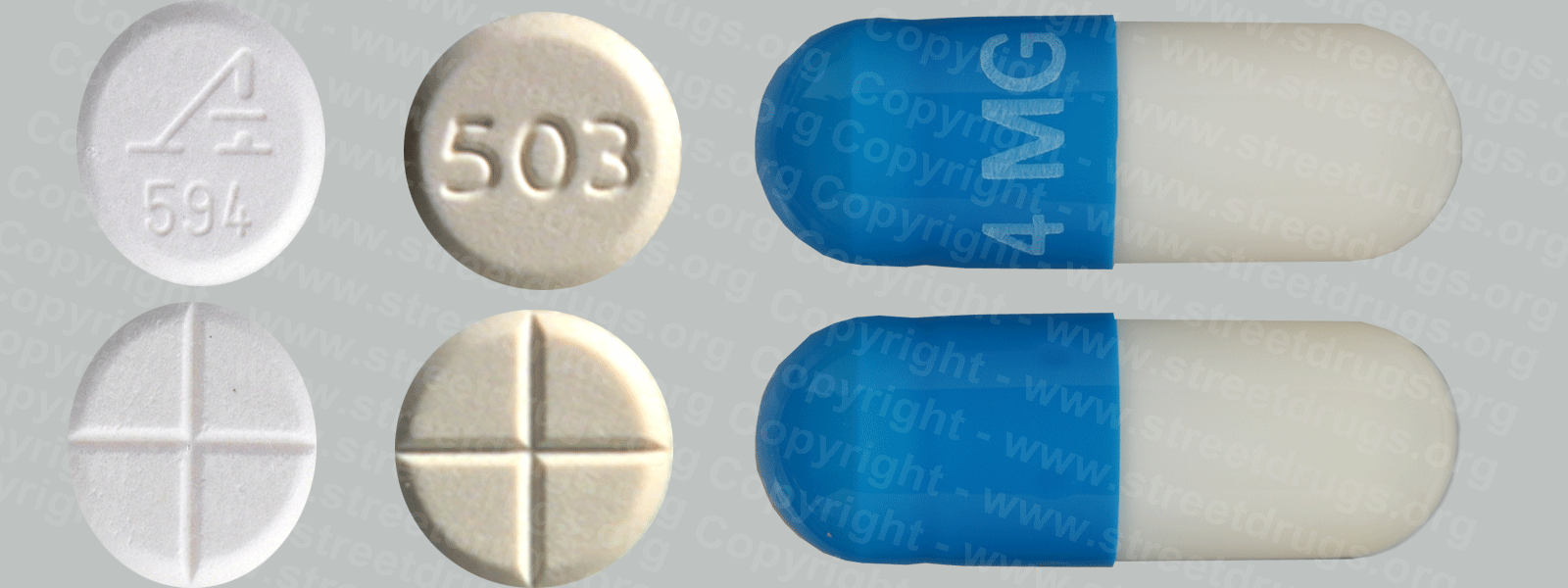
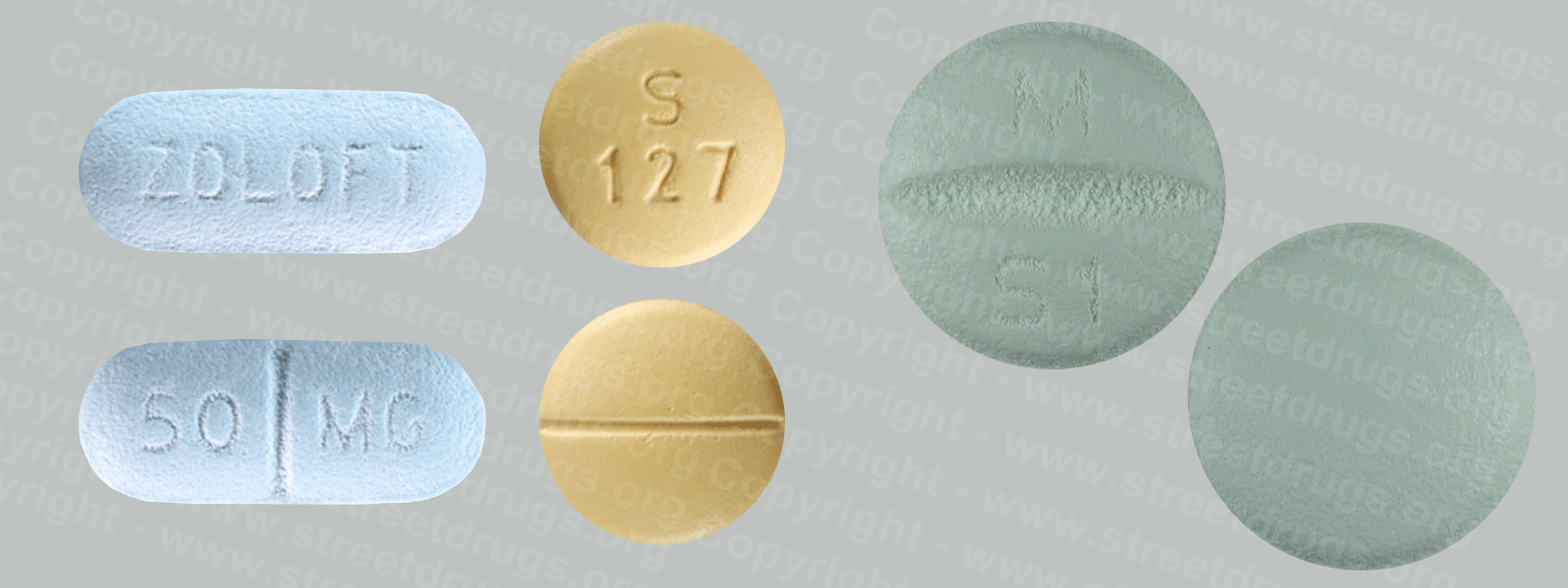
Detropropoxyphene
Dextropropoxyphene is associated with a number of toxic side effects and is among the top 10 drugs reported by medical examiners in drug abuse deaths.
DEXTROPROPOXYPHENE
A close relative of methadone, dextropropoxyphene was first marketed in 1957 under the trade name of Darvon®. Oral analgesic potency is one-half to one-third that of codeine, with 65 mg approximately equivalent to about 600 mg of aspirin. Dextropropoxyphene is prescribed for relief of mild to moderate pain. Bulk dextropropoxyphene is in Schedule II, while preparations containing it are in Schedule IV. More than 100 tons of dextropropoxyphene are produced in the United States annually, and more than 30 million prescriptions are written for the products. This narcotic is associated with a number of toxic side effects and is among the top 10 drugs reported by medical examiners in drug abuse deaths.
The Controlled Substances Act (CSA) regulates five classes of drugs: narcotics, depressants, stimulants, hallucinogens, and anabolic steroids. Each class has distinguishing properties, and drugs within each class often produce similar effects. However, all controlled substances, regardless of class, share a number of common features. It is the purpose of this introduction to familiarize the reader with some of these shared features and to give definition to terms (printed in bold) frequently associated with these drugs.
With the exception of anabolic steroids, drugs in the other classes are utilized to alter mood, thought, and feeling through their actions on the central nervous system (brain and spinal cord). For example, some of these drugs alleviate pain, anxiety, or depression. Some induce sleep and others energize. Though therapeutically useful, the “feel good” effects of these drugs contribute to their abuse. The extent to which a substance is reliably capable of producing intensely pleasurable feelings (euphoria) increases the likelihood of that substance being abused.
When drugs are used in a manner or amount inconsistent with the medical or social patterns of a culture, it is called drug abuse. In legal terms, the non-sanctioned use of substances controlled in Schedules I through V of the CSA is considered drug abuse. While legal pharmaceuticals placed under control in the CSA are prescribed and used by patients for medical treatment, the use of these same pharmaceuticals outside the scope of Sound medical practice is drug abuse.
In addition to having abuse potential, most controlled substances are capable of producing dependence, either physical or psychological. Physical dependence refers to the changes that have occurred in the body after repeated use of a drug that necessitates the continued administration of the drug to prevent a withdrawal syndrome. This withdrawal syndrome can range from mildly unpleasant to life-threatening and is dependent on a number of factors. The type of withdrawal experienced is related to the drug being used; the dose and route of administration; concurrent use of other drugs; frequency and duration of drug use; and the age, sex, health, and genetic makeup of the user. Psychological dependence refers to the perceived “need” or “craving” for a drug. Individuals who are psychologically dependent on a particular substance often feel that they cannot function without the continued use of that substance. While physical dependence disappears within days or weeks after drug use stops, psychological dependence can last much longer and is one of the primary reasons for relapse/initiation of drug use after a period of abstinence).
Contrary to common belief, physical dependence is not addiction. While addicts are usually physically dependent on the drug they are abusing, physical dependence can exist without addiction. For example, patients who take narcotics for chronic pain management or benzodiazepines to treat anxiety as compulsive drug-seeking behavior where acquiring and using a drug becomes the most important activity in the user’s life. This definition implies a loss of control regarding drug use, and the addict will continue to use a drug despite serious medical and/or social consequences. The National Institute on Drug Abuse (NIDA) estimates that about five million Americans suffer from drug addiction.
Individuals that abuse drugs often have a preferred drug that they use, but may substitute other drugs that produce similar effects (often found in the same drug class) when they have difficulty obtaining their drug of choice. Drugs within a class are often compared with each other with terms like potency and efficacy. Potency refers to the amount of a drug that must be taken to produce a certain effect, while efficacy refers to whether or not a drug is capable of producing a given effect regardless of dose. Both the strength and the ability of a substance to produce certain effects play a role in whether that drug is selected by the drug abuser.
It is important to keep in mind that the effects produced by any drug can vary significantly and is largely dependent on the dose and route of administration. Concurrent use of other drugs can enhance or block an effect and substance abusers often take more than one drug to boost the desired effects or counter unwanted side effects. This means that the risks associated with drug abuse cannot be accurately predicted because each user has his/her own unique sensitivity to a drug. There are a number of theories that attempt to explain these differences, and it is clear that a genetic component may predispose an individual to certain toxicities or even addictive behavior.
Youths are especially vulnerable to drug abuse. According to NIDA, young Americans engaged in extraordinary levels of illicit drug use in the last third of the twentieth century. Today, the majority of young people (about 55 percent) have used an illicit drug by the time they leave high school and about 25 percent of all seniors are current (within the past month) users. The behaviors associated with teen and preteen drug use often result in tragic consequences with untold harm to others, themselves, and their families. For example, an analysis of data from the National Household Survey on Drug Abuse indicates that youngsters between the ages of 12 and 17 who have smoked marijuana within the past year are more than twice as likely to cut class, steal, attack people, and destroy property than are those who did not smoke marijuana. The more frequently a youth smokes marijuana, the more likely he or she is to engage in these antisocial behaviors.
In the sections that follow, each of the five classes of drugs is reviewed and various drugs within each class are profiled. Although marijuana is classified in the CSA as a hallucinogen, a separate section is dedicated to that topic. There are also a number of substances that are abused but not regulated under the CSA. Alcohol and tobacco, for example, are specifically exempt from control by the CSA. In addition, a whole group of substances called inhalants are commonly available and widely abused by children. Control of these substances under the CSA would not only impede legitimate commerce but would likely have little effect on the abuse of these substances by youngsters. An energetic campaign aimed at educating both adults and youth about inhalants is more likely to prevent their abuse. To that end, a section is dedicated to providing information on inhalants. The last section in this publication is entitled U.S. Chemical Control. In recent years, a significant effort has been initiated by the United States to reduce the availability of clandestinely produced drugs by limiting the availability of chemicals and equipment needed to produce them. This section provides information on chemical control and specifically lists those chemicals that are currently regulated under the CSA.
Source: DEA
Other Sources
Whitehouse Drug-Free Communities